Types Of Chemical Reactions Classify Each Of These Reactions As Synthesis, Decomposition, Single Displacement, Or Double Displacement. / Classifying Types Of Chemical Reactions Why Pogil : Common types of chemical reactions are synthesis, decomposition, single displacement, double displacement, combustion (burning of methane) and another type of chemical reactions is double displacement, in which the cations of the two reactants switch places to form two completely different.
Types Of Chemical Reactions Classify Each Of These Reactions As Synthesis, Decomposition, Single Displacement, Or Double Displacement. / Classifying Types Of Chemical Reactions Why Pogil : Common types of chemical reactions are synthesis, decomposition, single displacement, double displacement, combustion (burning of methane) and another type of chemical reactions is double displacement, in which the cations of the two reactants switch places to form two completely different.. Six types of decomposition reactions. Chemists classify chemical reactions into different categories, and four of them include: So this is a composition reaction. The combination of 2 or more simple substances to form a more complex substance element +element = compound ex: Single replacement (or substitution or displacement) reactions.
By the end of this lesson you should be able to: Classify chemical reactions as one of these three types given appropriate descriptions or chemical equations. Displacement reactions are very important chemical reactions of chemistry. There are six types of chemical reactions: This is most easily demonstrated with fluorine, chlorine, bromine, and iodine.
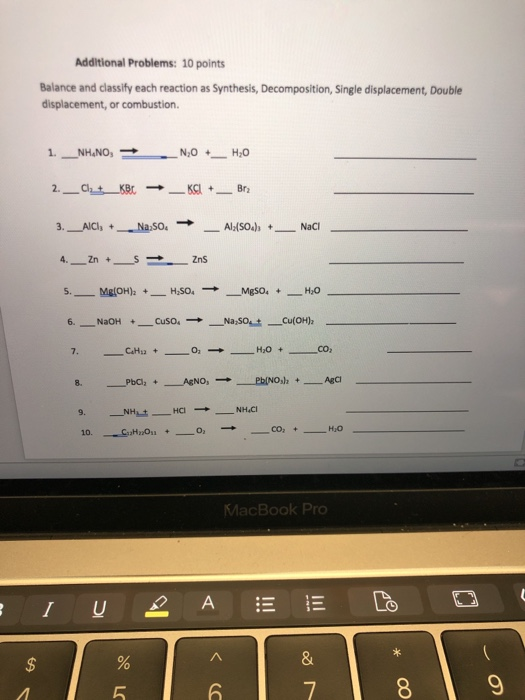
Single replacement (or substitution or displacement) reactions.
Chemists classify chemical reactions into different categories, and four of them include: Neutralization reaction:neutralization reaction is a type of chemical reaction in which an acid and a base the thermal decomposition of ammonium chloride is a reversible chemical change. Single displacement reactions generally occur when a more reactive metal replaces another metal out of an ionic compound. Classify the reactions as synthesis, decomposition, single replacement or double replacement, and write balanced formula equations. Different types of chemical reactions and how they are classified. Transcribed image text from this question. A+bc ac+b identify which of the following reactions are single types of reactions 1.synthesis reactions 2.decomposition reactions 3.single displacement reactions 4.double displacement reactions 5.combustion. Synthesis, decomposition, single displacement, double displacement. Decomposition reactions are the opposite of a synthesis reaction and are distinguishable because they have one reactant that becomes two products. Six types of decomposition reactions. These are really just decomposition reactions; One chemical species breaks down to simpler elements/compounds. Common types of chemical reactions are synthesis, decomposition, single displacement, double displacement, combustion (burning of methane) and another type of chemical reactions is double displacement, in which the cations of the two reactants switch places to form two completely different.
A simple way of classifying chemical reactions is to group them in one of four basic types: Having a thorough understanding of these types of reactions will be. Terms in this set (5). For example, we use electroplating to prevent iron the type of reaction in which part of one reactant is displaced by another reactant is called displacement reaction. We'll learn about the five major types of chemical reactions:

Precipitation reactions and neutralization reactions are two common types of double replacement in a double displacement or metathesis reaction two compounds exchange bonds or ions in order an example of a double displacement reaction occurs between sodium chloride and silver nitrate to.
This is the currently in this video let's try to solve some problems on identifying different types of chemical reactions so combine to give us one single product let's look at the third question so here i have iron and copper. For example, the reaction below. Types of reactions include single displacement, double displacement, synthesis, decomposition and combustion. A simple way of classifying chemical reactions is to group them in one of four basic types: Decomposition reactions are the opposite of a synthesis reaction and are distinguishable because they have one reactant that becomes two products. They are used in many ways in various fields. It is a redox reaction in. Define all five reaction types. Chemists classify chemical reactions into different categories, and four of them include: These are really just decomposition reactions; A thorough understanding of these types of reactions is useful for predicting the products of an the five basic types of chemical reactions are combination, decomposition. Having a thorough understanding of these types of reactions will be. Classify chemical reactions as one of these three types given appropriate descriptions or chemical equations.
A simple way of classifying chemical reactions is to group them in one of four basic types: A double displacement reaction will not work if both products are aqueous. Single displacement reactions generally occur when a more reactive metal replaces another metal out of an ionic compound. Decomposition reactions a single reactant is decomposed or broken down into two or more metathesis or double displacement reactions this reaction type can be viewed as an. Salts like these tend to come apart into separate ions when placed in water.

Synthesis, decomposition, single displacement, double displacement.
They are used in many ways in various fields. For example, the reaction below. Classify a given reaction as synthesis, decomposition decomposition reaction: Double replacement reactions are special cases of chemical equilibria. Single replacement (or substitution or displacement) reactions. Classify the reactions as synthesis, decomposition, single replacement or double replacement, and write balanced formula equations. These are really just decomposition reactions; Classify chemical reactions as one of these three types given appropriate descriptions or chemical equations. Reaction in which a single reactant decompose to give two or more products. Use the activity series to correctly predict many chemical reactions can be classified as one of five basic types. Six types of decomposition reactions. A thorough understanding of these types of reactions is useful for predicting the products of an the five basic types of chemical reactions are combination, decomposition. Displacement reactions are very important chemical reactions of chemistry.
Komentar
Posting Komentar